UP scientists develop new test for designer drugs
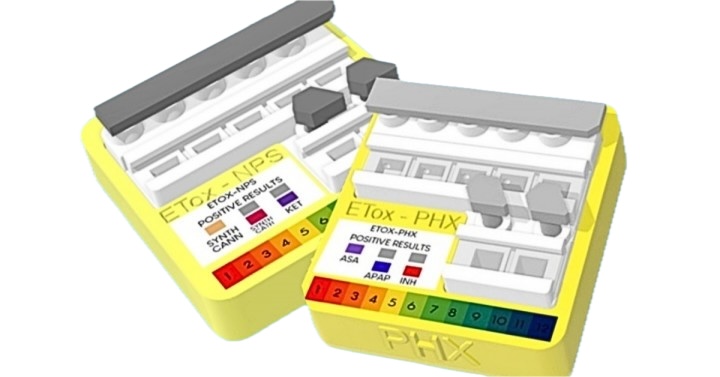
Researchers from the University of the Philippines Manila have developed a new test kit that can be used to detect selected New Psychoactive Substances (NPS), more commonly known as “designer drugs,” and other pharmaceuticals, which cause poisoning.
The “E-Tox POCT (Point of Care Testing)” test kit uses urine samples and is available in two models: E-Tox-NPS, which can detect designer drugs such as ketamine, synthetic cannabinoids and synthetic cathinones; and E-Tox-PHX, which looks for pharmaceuticals such as paracetamol (a popular medicine for mild to moderate pain), isoniazid (an antibiotic used against tuberculosis) and salicylates (including aspirin, a commonly used drug for pain and fever).
Dr. Ailyn Yabes, the project leader, who is also a professor at the UPM-College of Medicine’s Department of Pharmacology and Toxicology, said there was a need for “a portable, user-friendly and cost-effective point-of-care testing device in emergency settings.”
“Existing testing platforms for pharmaceuticals and NPS detection are often lab-based, costly and require hazardous chemicals, making them inaccessible and impractical for emergency or bedside toxicology,” she added.
At the moment, the country has no test kits that detect NPS. “The E-Tox-NPS will be very useful because there is no POCT screening kit for NPS available for use [here]. NPS is also not yet included in the drugs of abuse panel in the Philippines. Local screening tests are limited to traditional illicit drugs such as amphetamine and cannabinoids,” Yabes said.
She pointed out, however, that E-Tox-NPS should be used only for “screening”—an initial test to check for the presence of designer drugs. A more specific and sensitive method will be needed to confirm the presence of NPS in the urine, she added.
Low cost
Yabes said the final cost of the prototype E-Tox kit, excluding labor, was P88.10. Current drug tests conducted in laboratories range from P200 to P1,000 but these screen only for traditional illegal drugs such as amphetamine (found in shabu) and cannabinoids (found in marijuana). A urine test may cost from between P110 in public hospitals to as much as thousands of pesos for a complete package of procedures.
The test kits have an estimated shelf life of eight months as long as the recommended storage conditions are followed.
Both models have undergone validation and demonstrated acceptable performance characteristics in terms of limit of detection, accuracy, precision, sensitivity, specificity and predictive values based on standards set by the United Nations Office on Drugs and Crime (UNODC), Clinical and Laboratory Standards Institute and International Organization for Standardization.
Top cause of poisoning
According to data gathered by the UP-Philippine General Hospital (PGH) National Poison Management and Control Center, pharmaceutical agents remain the top cause of poisoning, accounting for 35.5 percent of in-patient referrals.
“Paracetamol is the main cause of poisoning, not only accidentally but also nonaccidentally or deliberately,” Yabes said, noting that paracetamol poisoning is a pressing issue worldwide.
NPS abuse is also a growing problem in different parts of the world, with authorities having to keep up with new regulations for the clandestinely sold substances. The UNODC reported that 1,242 NPS were in circulation while data from the Philippines showed that out of 74 samples for NPS poisoning, 41 cases involved NPS, with cathinones being the predominant class.

Multiagency backing
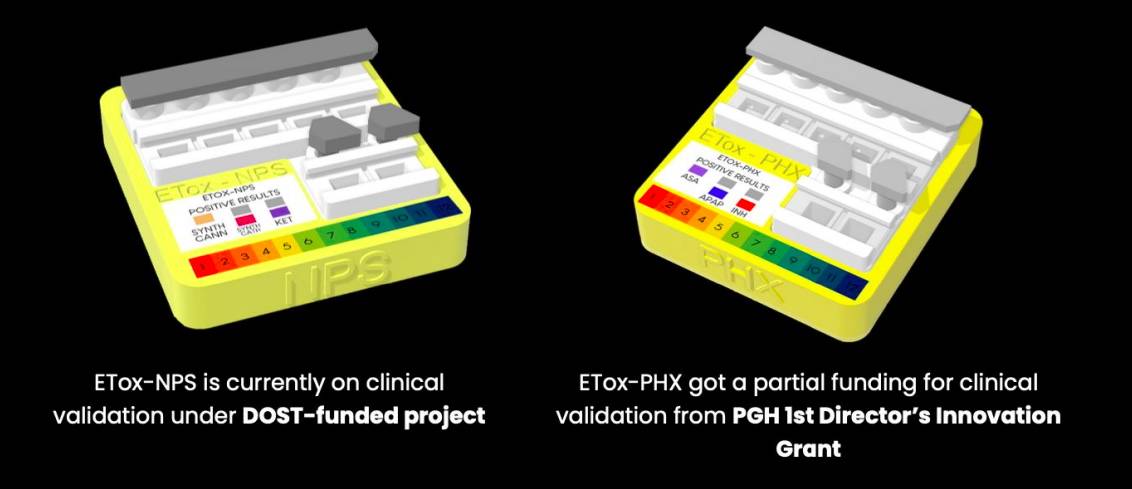
The development of the test kits was supported by various funding agencies, including the Dangerous Drugs Board, Department of Science and Technology –Philippine Council for Health Research and Development and the UP-PGH 1st Director’s Innovation Grant.
Clinical validation for E-Tox-NPS is expected to be concluded by October while it is ongoing for E-Tox-PHX. The developers have applied for a patent with Intellectual Property Office.
Yabes said the research team was looking for partners for the manufacturing and commercialization of the portable drug test kits. They are also in need of additional funding for clinical validation studies and the development and optimization of injection molded prototypes.
Aside from Yabes, other members of the team who developed the test kits are UPM professors Dr. Noel Quiming and Sarah Johnson as co-investigators; and university researchers Gabrielle Pagjunasan, Greg Andrew Octa and Jessa Turreda as project staff. INQ

















